Local and National Increases:
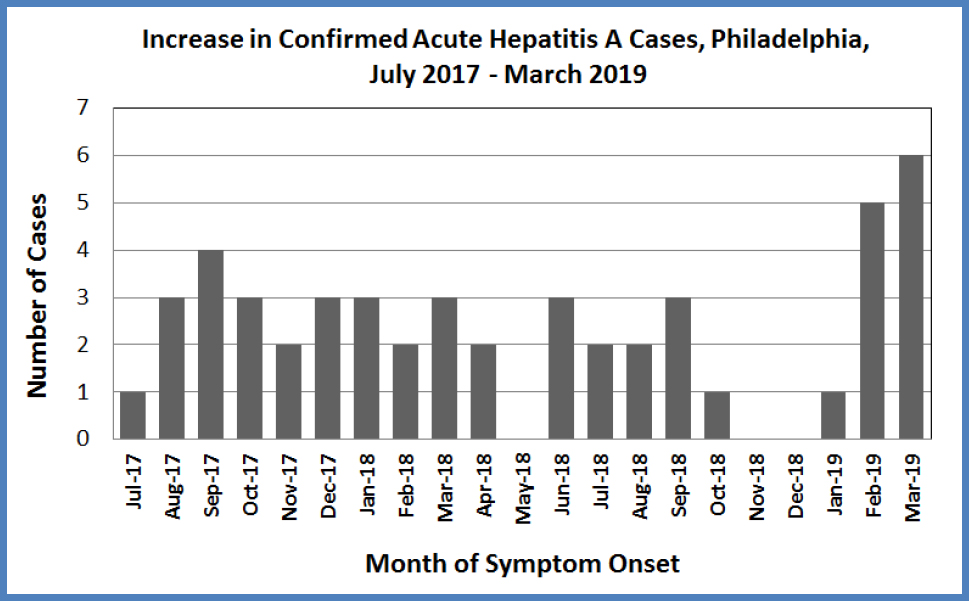
Across the US, there are outbreaks of hepatitis A in several states, especially among unvaccinated, adult populations at high risk (illicit drug users, homeless persons, and men who have sex with men [MSM]). Reflecting this trend, 49 confirmed HAV cases have occurred in Philadelphia since July 2017, which is a marked increase from a median of 6 cases reported annually from 2012–2016 (range: 2–9 cases). All but one case was 17 years of age or older, and 80% of the cases were hospitalized. While most of the outbreaks in other states have occurred among high risk groups, many Philadelphia cases do not have clear risk factors. In the first quarter of 2019, slightly higher levels of Hepatitis A activity have been reported in the City (N=12, see graph below), and an infected food handler at an area grocery store was recently identified (see press release). The Philadelphia Department of Public Health (PDPH) is issuing this Health Alert to increase recognition of cases, and to emphasize prevention through vaccination to persons at high risk and to all patients seeking vaccine.
HAV Diagnosis and Control:
Prompt recognition of HAV is important to control transmission and allow time-sensitive administration of post-exposure prophylaxis (PEP) to exposed contacts. Providers should consider HAV for any patient who presents with fever, fatigue and signs of liver damage: dark urine, clay colored or pale stools, jaundice, abdominal pain, nausea and vomiting. To confirm infection, suspected, symptomatic cases should have serum sent for HAV IgM and liver function tests.
HAV Infection Control:
The Philadelphia Department of Public Health (PDPH) encourages area healthcare providers who have diagnosed or are providing care to a patient with acute HAV to:
- Educate HAV cases that the virus is shed in their stool from 2 weeks before through 1 week after their jaundice onset and can be spread to others through close person-to-person contact or contamination of food or other objects with even tiny amounts of stool from a person with HAV.
- Advise cases to properly wash hands after using the bathroom, limit food preparation for others, and avoid sex while contagious. Sexually active persons should use latex barrier methods, such as dental dams or condoms to help prevent transmission and wash hands, genitals, and sex toys before and after sex.
- Instruct HAV cases who work in high risk settings (i.e., food service, daycares, healthcare) to remain out of work for 1 week after jaundice onset or if no jaundice, 2 weeks after symptom onset.
- Recommend HAV PEP for close contacts (i.e., household members, sex partners). One dose of single antigen HAV vaccine given within 2 weeks of the last exposure can be used to prevent infection in healthy contacts aged 12 months and older who lack HAV immunity (2 prior doses of vaccine or disease history). HAV immune globulin can be used for exposed contacts who are immunocompromised or have HAV vaccine contraindications. HAV PEP is covered by many insurance plans. Call PDPH at 215-685-6742 for assistance with PEP access and administration.
HAV Vaccination:
Safe and highly effective HAV vaccines are available for persons aged 12 months and older to prevent infection. Given recent HAV increases, PDPH encourages providers to routinely vaccinate patients in accordance with the Advisory Committee on Immunization Practice recommendations (2 doses given 6–18 months apart). Populations recommended to receive HAV vaccine include:
- Routinely for children at age 12–23 months
- Any person wishing to obtain immunity
- International travelers to areas with high or intermediate hepatitis A endemicity
- Men who have sex with men
- Users of injection and non-injection drugs
- Persons experiencing homelessness
- Persons with chronic liver disease
- Persons with clotting factor disorders
- Persons who work with HAV-infected primates or with HAV in a research laboratory setting
- Persons who anticipate close contact with an international adoptee from a country of high or intermediate endemicity
For more information, please see: https://www.cdc.gov/mmwr/volumes/68/wr/mm6806a6.htm.
PDPH Vaccines for Children (VFC) and Vaccines for Adults at Risk (VFAAR) providers should continue to order HAV needed for your patients through the PhilaVax inventory module. If you have any questions about accessing HAV vaccine as a VFC/VFAAR provider, contact Jillian Brown at [email protected].
Reporting and Consultation:
All confirmed and suspected Hepatitis A infections should be promptly reported to PDPH at 215-685-6740 during regular business hours or 215-686-4514 after-hours. PDPH can provide details on local risk factors and exposures, diagnostic testing procedures, and recommendations for disease control including vaccination.
Originally released by the Philadelphia Department of Public Health on April 11, 2019.




